posted by Niamh Allen
November, 14th, 2024
Company News Environmental
Bioprocessing sits at the heart of the pharmaceutical manufacturing industry and has advanced scientific understanding of disease as well as aiding in the development of new medicines.
But what exactly is bioprocessing and how are bioprocess containers used during the forming of medicines, vaccines, and other drug products?
Continue reading to find out more!
How bioprocessing aids in developing new medicines
Bioprocessing is an essential manufacturing process when developing new medicines and pharmaceuticals to combat diseases, infections, and illnesses.
What is bioprocessing?
The process of bioprocessing involves using and manipulating living organisms, cells, or viruses to produce useful and desired products. In biopharmaceutical manufacturing, this includes drug products such as vaccines, antibiotics, and cell therapies, among others. .
Bioprocessing can be separated into two key stages: upstream and downstream processing.
Upstream processing
Upstream processing is the first stage of bioprocess manufacturing and involves the preparation and optimisation of cell cultures and other biological materials for catalytic reactions.
Upstream processing is essential to the overall quality, quantity, and potency of the final drug product, so meticulous procedures need to be followed to ensure this, including the safe storage of biological fluids in bioprocess containers – but we’ll get to this later.
Upstream processing can include:
- Media preparation – the formulation of nutrient-rich substances to aid the growth of microorganisms.
- Inoculation – the introduction of microbes into the liquid media or agar plate so they develop and multiply.
- Cell culture – the growth of cells or yeast in a controlled environment, such as an incubator.
- Fermentation – the addition of nutrients at the correct concentration into a bioreactor, also known as a fermentor, to enhance the productivity of the harvested organisms.
Downstream processing
Downstream processing refers to the recovery and purification stage of formulating pharmaceutical products to be manufactured in large quantities. It consists of the following steps:
Purification – the process of separating the desired protein from the other products or contaminants formed during fermentation. This is essential for ensuring the drug’s purity reaches between 98% to 100%.
Harvesting – the separation or isolation of cells from growth media using centrifugation, filtration, flocculation, or sedimentation methods. This step, if done correctly, will maximise the yield and quality of new medicines.
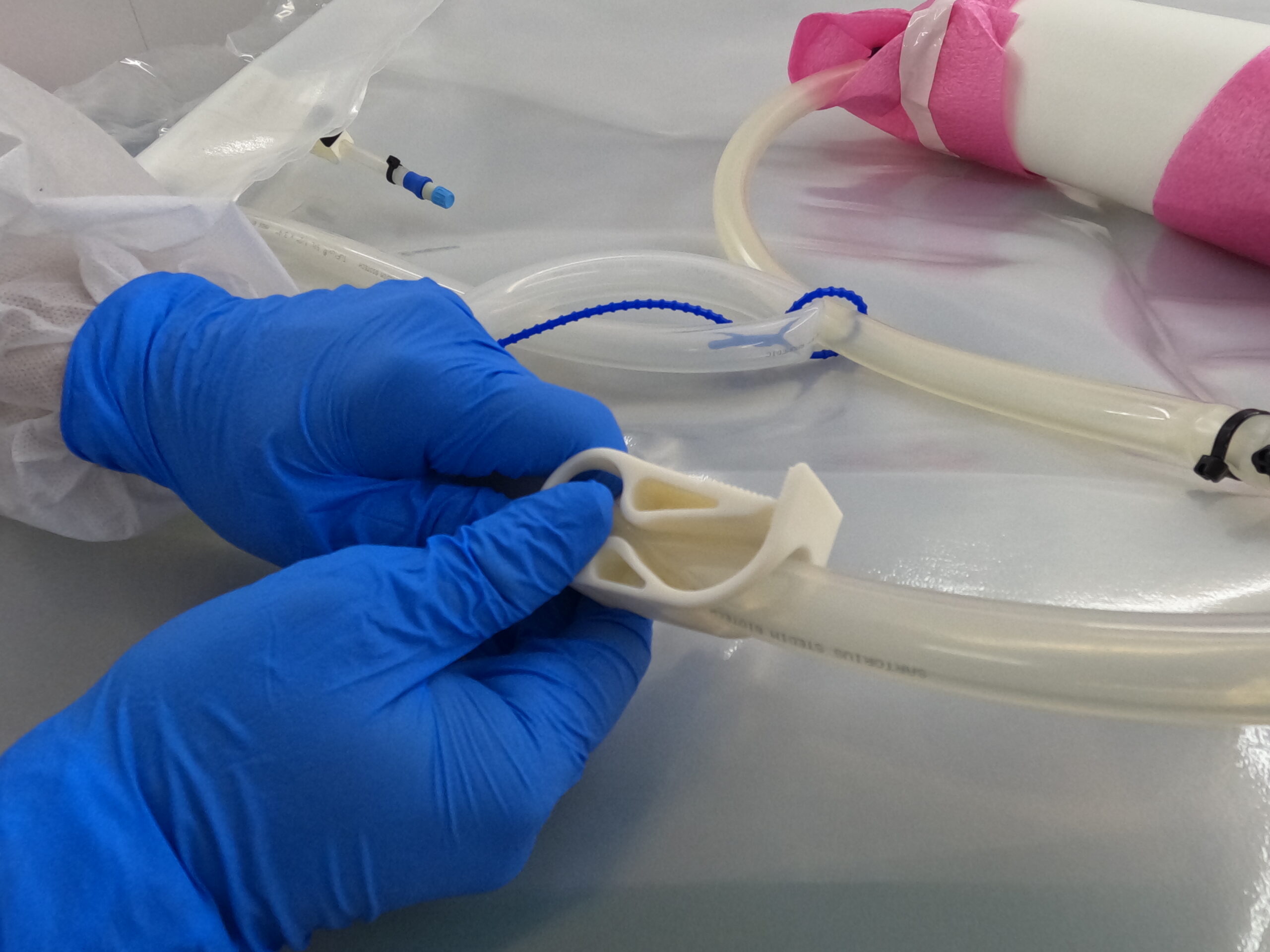
The importance of bioprocess containers
Bioprocess containers are vital fluid storage systems used throughout the entire bioprocess workflow, from media preparation to harvesting, and virtually any other liquid handling process involved in the creation of new medicines.
These systems are designed to safely store single-use bags for cleanroom applications and whilst in transit between facilities. Furthermore, bioprocess containers help to prevent bacterial contamination and leakages that could compromise research and drug development.
Typically, most bioprocess containers are made from flexible film and poly materials and are disposed of after one single use.
Bioprocessing solutions that these containers are designed to house include:
- Product samples
- Buffer solutions
- Bioreactor feed
- Cell and tissue culture mediums
- Chromatography feed
- Fraction collection
Why choose ALLpaQ containers?
Unlike single-use bioprocess containers, our reusable systems help to streamline your pharmaceutical development and research practices and save you money in the long run.
With simple bag placement via a handy drop door and sliding door access to tubing, you won’t look back after upgrading to reusable ALLpaQ cleanroom storage and shipping containers.
Benefits of ALLpaQ containers
- Eliminate contamination potential
Consisting of a hard-wearing polypropylene shell, our reusable bioprocessing containers resist impacts and corrosion, helping to keep delicate media, sensitive biomaterials, and buffers safe from external impacts and contamination during transportation or storage.
This differs from single-use systems that can tear when unfolding.
What’s more, your bioprocessing container will be easier to clean thanks to smooth removable panels that are suitable for jet washing, alleviating the potential for bacterial growth.
- Say goodbye to repeat orders
One bioprocess container from our extensive collection is built to last for numerous years, saving you the costs and hassle of ordering repeat orders of single-use systems.
Repeatability also helps to reduce plastic waste, boosting your company’s sustainability practices and commitment to greener environmental practices.
With ergonomic palleted bases, these units can be stacked conveniently in warehouses, saving storage space around your facility.
- Customisable container systems
Our innovative fluid-handling storage units can be custom-made to ensure your liquid management system equipment is precisely aligned with your needs.
To improve your sustainable practices and warehousing, as well as provide the ultimate protection for your stored fluids during biopharmaceutical manufacturing, you should consider opting for collapsable and reusable bioprocess container systems.
Contact ALLpaQ today
If you want to make your laboratory more sustainable and reduce your research expenditure in the long run, we’re sure to have a fitting liquid storage solution for you.
At ALLpaQ, we’re a prestigious manufacturer of reusable bioprocess container systems and accessories, and if necessary we can use our expertise to design a bespoke system for your individual requirements.
To enquire further or to discuss custom biocontainers, don’t hesitate to call us on 01472 800 373 or email us at enquiries@allpaq.com and we’ll be in touch ASAP.e or call on 0147 2800 373.
TAGS:
ALLpaQ, ISO14001, sustainability,
SHARE:
Author
Niamh Allen
As a dedicated and detail-oriented Marketing Assistant, Niamh brings a strong passion for creativity and analytics to the marketing team.