posted by Phill Allen
April, 04th, 2014
Company News Pharmaceutical Industry News
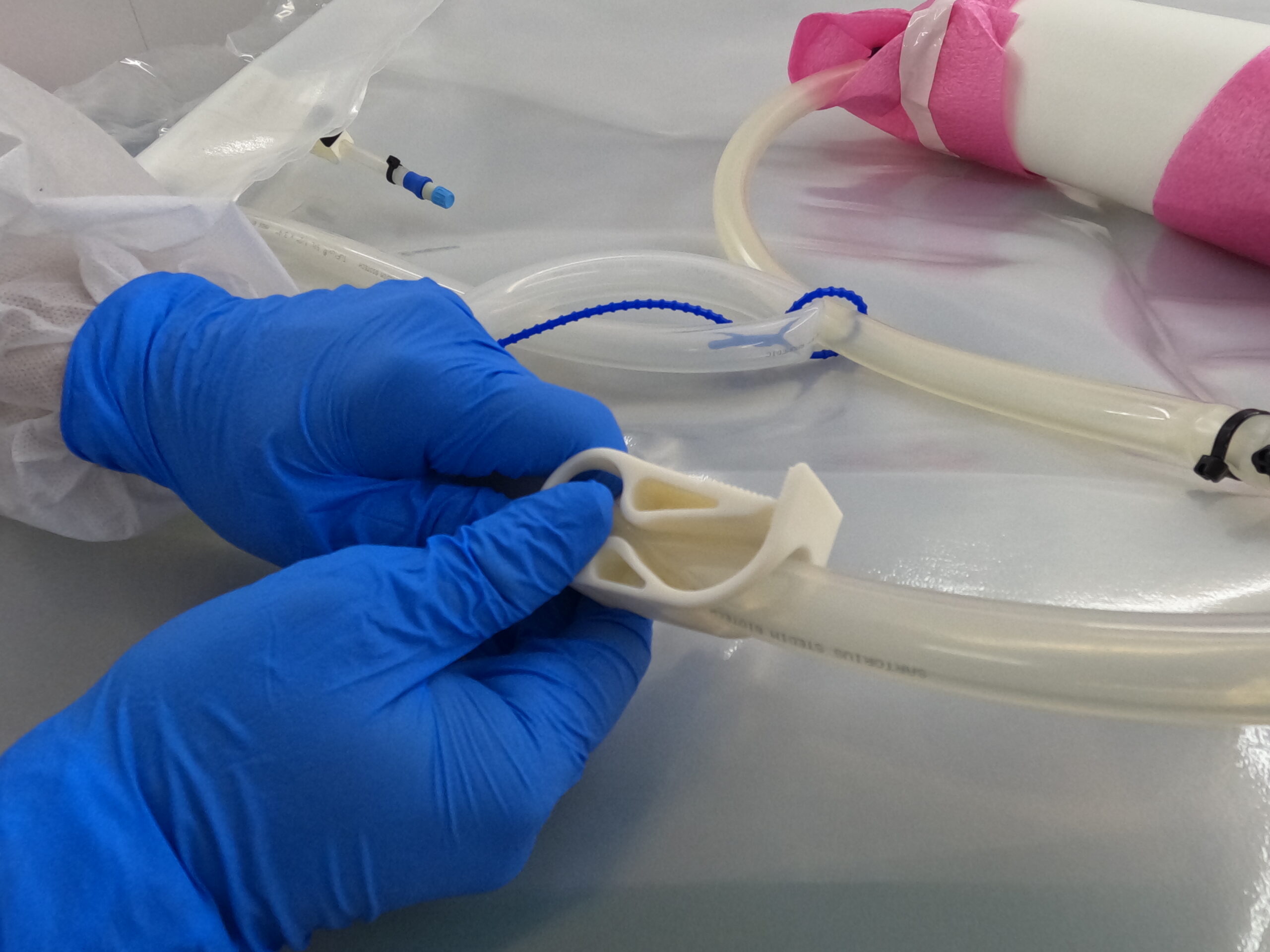
 AIR is the newest member of the ALLpaQ bioprocess containment product family. The product, which was launched earlier in 2014, optimises the air transportation of biopharmaceutical media held under temperature controlled conditions in the Envirotainer RAP e2 container.
AIR is the newest member of the ALLpaQ bioprocess containment product family. The product, which was launched earlier in 2014, optimises the air transportation of biopharmaceutical media held under temperature controlled conditions in the Envirotainer RAP e2 container.
In effect, AIR bioprocess containers allow companies to double the load during airfreight, generating huge cost savings and operational efficiencies.
Prior to take off, AIR went through a rigorous process of independent structural and transportation tests conducted by Smithers Pira in the United Kingdom in accordance with ASTM D4169-09, Distribution Cycle 12 for air and motor freight, Assurance Level II.
We caught the footage on tape and thought you might want to take a look.
Related reading:
TAGS:
air cargo, ALLpaQ AIR, Envirotainer, Plastic Bioprocess Containers,
SHARE:
Author
Phill Allen
Managing Director
Phill is an innovative thinker particularly in fluid management. His expertise lies in ensuring the seamless flow of pharmaceutical liquid logistics, whether it's optimising current processes or pioneering new approaches.