Pharmaceutical Industry News
Downstream vs upstream processing
Upstream and downstream processing work in tandem to produce desirable products, such as beneficial pharmaceuticals like medicines or biochemicals. But […]
Oct 21st, 2025

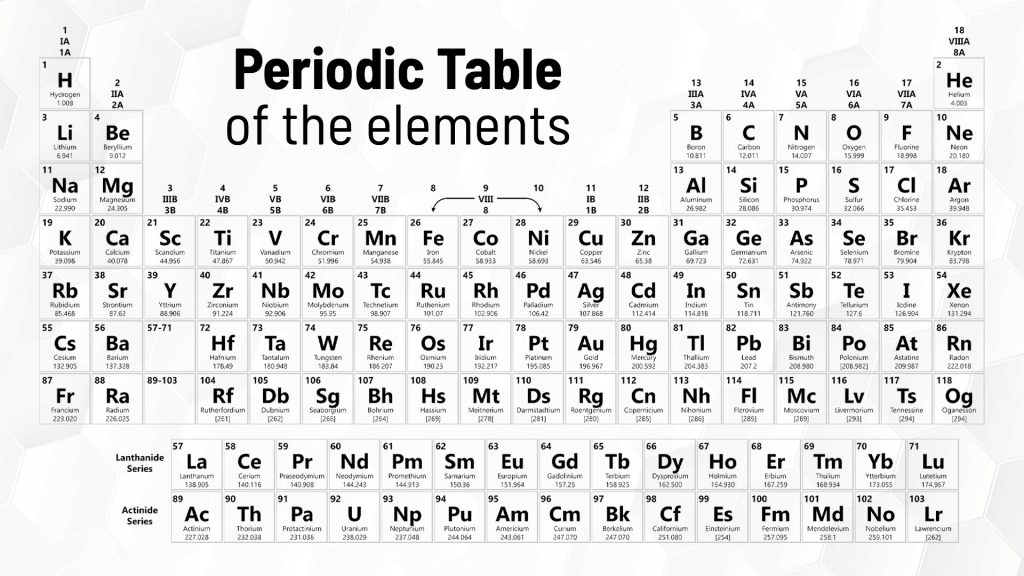
The Periodic Table is a fundamental tool in chemistry, both in its application and its teaching. The Periodic Table helps us understand the properties of elements and their behaviour, particularly in reference to how they relate to one another.
Put simply, the Periodic Table organises elements according to the similarity of their properties, so scientists are able to tell the characteristics of an element just by looking at its location on the table. Each element shares characteristics with those next to it on the table. Also, a good understanding of the Periodic Table can come in really useful if you’re a member of a pub quiz team!
As a leading provider of pharmaceutical products to the biopharma sector, we at ALLpaQ understand the importance of chemistry in advancing the discovery, development, and manufacture of new drugs and therapies.
We think that the development of the Periodic Table is a good example of scientific best practice, as it was a collaborative endeavour, which benefited from a lot of inspiration and good old-fashioned research. The table also evolved as our understanding grew, just like any scientific theory or process must as our knowledge improves.
As with so many scientific discoveries, the history of the Periodic Table isn’t the story of one person’s achievements, but rather an accumulation of many generations of developing understanding.
The Royal Society of Chemistry makes the case that the earliest attempt to classify the elements was completed in 1789 by one Antoine Lavoisier – a French nobleman, revolutionary and chemist whose work was cut tragically short in 1794 by Madame Guillotine. Lavoisier identified and named both oxygen and hydrogen and wrote the first extensive list of elements, which he grouped into gases, non-metals, metals and earths, based on their properties.
In 1803, Manchester’s own John Dalton formulated a new theory to explain the absorption of gases by liquids. He suggested that each element consists of its own unique arrangement of indivisible atoms. As Manchester’s Science and Industry Museum reports: Dalton theorised that atoms of one element are all alike, but they are different from the atoms of other elements. To illustrate this, Dalton calculated atomic weights for the atoms of the 20 elements he knew about. This was revolutionary thinking at the time, and would lead directly to the development of the Periodic Table.
By the 1860s, Dmitri Mendeleev was a brilliantly unconventional Russian scientist who, for example, couldn’t find a good enough book in Russian about chemical principles, so wrote one.
Mendeleev certainly wasn’t the only scientist working on arranging elements in order to understand the connections between them, however, in 1869 he created what we now recognise as the Periodic Table (or Periodic System, as he called it).
He developed his system using the delightfully analogue method of writing the properties of each element on a card and arranging those cards, then rearranging them, until patterns began to emerge. He realised that, by putting them in order of increasing atomic weight, a reactive non-metal was directly followed by a very reactive light metal and then a less reactive light metal.
His real masterstroke was his decision to leave gaps in the table – because he correctly theorised there would be more elements to discover, which would fit into this same structure. This is why the table has proven so robust and is still employed today, because it can be updated as our knowledge improves.
Mendeleev’s methodology is called ‘Periodic Law’ and, using it, he correctly identified where several yet-to-be-discovered elements would sit on the table.
Indeed, part of the reason for the continuing usefulness of the Table is that it has allowed generations of scientists to predict the properties of the elements that were yet to be discovered.
For example, in the 1880s, during Mendeleev’s lifetime, three more elements were discovered and his predictions about where they would fit on the Table were proven to be accurate.
Mendeleev was never recognised by the Nobel Academy for his groundbreaking work, but he does have a far rarer distinction: Element 101 was named ‘Mendelevium’ after him.
In 1913, Henry Moseley’s work on X-ray spectroscopy successfully explained why the Table worked. Mendeleev arranged the elements by atomic mass, which usually produced the correct order, but not in every case. Moseley used his newly-developed X-ray gun to actually measure atomic numbers. This confirmed Mendeleev’s findings and explained the anomalies in the original Table.
Tragically, his work was cut short when he bravely declined a position as a professor at Oxford in order to join the Royal Engineers and fight on the Front Line. He was killed by a sniper in Turkey, in August 1915.
The Periodic Table now consists of no fewer than 118 elements, each with its own unique properties and characteristics. Elements are organised into seven periods and eighteen groups and arranged in relation to each other. So, for example, as you move from right to left on the Table, the size of the atoms in the element decreases while the ionisation energy increases.
Understanding these properties and their relationship with those of the elements around them, allows chemists to identify trends and patterns as well as to predict chemical reactions.
The significance and application of this understanding goes beyond the world of pure chemistry and, for example, plays an important role in the pharmaceuticals and biopharma industries, as understanding the chemical behaviours and interactions of elements is crucial, since even small variations in chemical structure can have profound effects on drug efficacy and safety.
The periodic table has come a long way since its inception, evolving from a simple arrangement of elements to a comprehensive guide to the building blocks of matter. Its impact on the field of chemistry and various scientific disciplines cannot be overstated.
But. like all good scientific practice and products, the Table has evolved over the years, to keep it relevant and up to date.
Here at ALLpaQ, we’re aware that science can’t stand still, that’s why our cleanroom and shipping containers, as well as our 2D bag handling accessories, are always being improved. This is so we can maintain our status as the world’s premier supplier of plastic pharmaceutical products.
We do this by always ensuring that we are listening to the changing needs of our clients and developing solutions to their problems.
If you’d like to know more about our range of ever-evolving biocontainers and associated products, just get in touch by filling out the form, below.